Press Release
Cloom Tech Delivers Molex Cable Assemblies for High-Performance Electronics
Medley, FL 33178, United States, 14th Nov 2025 – Cloom Tech, an industry-leading manufacturer of custom wire harnesses and cable assemblies, has announced the successful delivery of a specialized series of Molex cable assemblies developed for advanced electronic applications. The assemblies are designed to provide enhanced electrical performance, mechanical reliability, and long-term stability across demanding industrial environments.

The introduction of these Molex assemblies reflects Cloom Tech’s focus on precision manufacturing and tailored connectivity solutions. Each assembly is produced to support complex configurations used in automotive engineering, robotics, aerospace systems, and medical devices. Through comprehensive design validation and controlled production, the assemblies meet stringent requirements for signal integrity, environmental endurance, and component compatibility.
The production process behind the Molex assemblies emphasizes precision at every stage. Material selection, connector integration, and quality verification are coordinated through a standardized system of inspection and testing. The result is a set of wiring solutions engineered to deliver stable electrical pathways under variable operating conditions, including temperature fluctuations, vibration, and electromagnetic exposure.
“The development and delivery of Molex cable assemblies mark a strategic step toward providing reliable and adaptable connectivity solutions for high-performance electronics,” stated Ivy Zhao, Spokesperson at Cloom Tech. “Each assembly is built through a meticulous process that ensures dimensional accuracy, consistent conductivity, and compliance with applicable performance standards.”
Cloom Tech’s engineering team applies advanced assembly methods to maintain uniformity across both low-volume prototypes and large-scale production runs. Rigorous testing procedures are employed to verify insulation resistance, continuity, and mechanical strength. Each product undergoes a full quality assessment before shipment, confirming adherence to international safety and reliability benchmarks.

The Molex assemblies are designed to support critical functions in systems requiring compact, efficient, and durable interconnections. In automotive and robotics applications, the assemblies contribute to dependable signal communication between controllers, actuators, and sensors. Within the aerospace sector, they support optimized performance in weight-sensitive environments, where precision wiring is essential to operational safety. Medical equipment manufacturers benefit from the assemblies’ precision and compliance with material and electrical standards that govern life-supporting technologies.
Beyond immediate functionality, the assemblies address broader industrial needs for modularity and adaptability. By combining standard connector configurations with customized wire specifications, Cloom Tech ensures that each solution integrates seamlessly into complex system architectures. This approach supports both existing designs and next-generation electronic innovations.
Cloom Tech’s Medley, Florida facility operates under strict process controls that govern material sourcing, assembly, and inspection. The company’s quality management framework ensures traceability from design concept through to final verification. Continuous improvement initiatives are integrated into production workflows to enhance manufacturing efficiency and product consistency.
In line with the company’s focus on technical precision, the development of the Molex assemblies also reflects an understanding of emerging industry trends. Increasing automation, miniaturization, and energy efficiency have created new challenges in electronic system design, and the assemblies are positioned to meet these challenges through adaptable engineering and robust construction.
“Future initiatives will continue to advance the capabilities of cable assembly manufacturing, focusing on innovation, material optimization, and automation,” Zhao added. “As industries progress toward higher data transmission speeds and more compact designs, Cloom Tech remains committed to delivering solutions that align with these evolving technical requirements.”
The release of the Molex assemblies underscores Cloom Tech’s role as a supplier of engineered connectivity products built for long-term performance and dependability. Each project undertaken by the company integrates detailed design collaboration, precise assembly, and verified quality assurance. By maintaining strict control over each stage of production, the organization ensures consistency in output and reliability in service.
Through continuous investment in technology, training, and inspection systems, Cloom Tech strengthens its position as a trusted manufacturer within the electrical and electronic supply chain. The Molex assembly initiative reflects a commitment to developing practical, high-quality solutions for industries that depend on accurate electrical transmission and durable design integrity.
For more information about Molex cable assemblies, please contact Cloom Tech at their office located at 9251 NW 112th Ave, Medley, FL 33178, USA. To inquire further, call +1 863 434 8447 or email sales@cloomtech.com.
Media Contact
Organization: Cloom Tech
Contact Person: Ivy Zhao
Website: https://cloomtech.com/
Email: Send Email
Contact Number: +18634348447
Address:9251 NW 112th Ave
City: Medley
State: FL 33178
Country:United States
Release id:37066
The post Cloom Tech Delivers Molex Cable Assemblies for High-Performance Electronics appeared first on King Newswire. This content is provided by a third-party source.. King Newswire makes no warranties or representations in connection with it. King Newswire is a press release distribution agency and does not endorse or verify the claims made in this release. If you have any complaints or copyright concerns related to this article, please contact the company listed in the ‘Media Contact’ section
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Top Plastic Surgeon Chicago Dr. Otto Placik Showcases Alloclae Innovation on WGN News
Dr. Otto Placik discusses the role of Alloclae as a non-surgical regenerative solution for breast cleavage enhancement, softening implant outlines, and hip dip correction.
Chicago, Illinois, United States, 27th Jan 2026 – Dr. Otto J. Placik, board-certified plastic surgeon and founder of BodySculptor.com, was recently featured on WGN News discussing the innovative role of Alloclae (AloeClae structured adipose matrices) in plastic surgery. The segment focused on how this regenerative technology is being used for isolated, non-surgical enhancement of breast cleavage, softening of breast implant outlines, correction of hip dips, and treatment of contour irregularities, without conventional surgery.
Alloclae is not a mommy makeover or surgical procedure. It is a non-surgical regenerative treatment designed to be used independently for targeted aesthetic correction.
Dr. Placik is the first board-certified plastic surgeon in Illinois to offer the Alloclae procedure, making his practice the earliest board-certified provider of this technology in the state. His practice is widely recognized as home to the top plastic surgeon in Illinois and top plastic surgeon in Chicago, a distinction that continues to draw patients seeking the most advanced aesthetic care.
The WGN News feature introduced Alloclae as a biostimulatory option designed to complement the body’s natural tissue response and restore soft volume in areas that traditionally required surgery.

Modern Mommy Makeover: A Regenerative Surgical Approach
In today’s aesthetic landscape, the Modern Mommy Makeover represents a regenerative surgical approach to body rejuvenation that centers on natural, restorative transformation. Unlike traditional methods that rely exclusively on implants, this approach incorporates autogenous (self-derived) tissue and biostimulatory materials.
Alloclae is not part of the mommy makeover procedure and is not a surgical solution. It is offered separately as a non-surgical regenerative treatment for patients who do not wish to undergo surgery.
Developed by board-certified plastic surgeon Dr. Otto J. Placik, the Modern Mommy Makeover combines surgical precision with regenerative science. The goal is to restore proportion and confidence while maintaining a natural aesthetic.
Natural Chest Enhancement with Autogenous Fat Transfer
A central component of the surgical approach is autogenous fat transfer, which uses a patient’s own fat for breast enhancement without implants. Fat is harvested from areas such as the abdomen, waist, or thighs, purified, and transferred to the chest to restore volume and shape.
This method provides:
- Use of self-derived tissue
- Biostimulatory regenerative response
- Long-term structural support through modern fat-processing techniques
For patients seeking subtle refinement, fat transfer may also be combined with a lift or a small implant to maintain balance and proportion.
Sculpting Beyond the Waistline
The Modern Mommy Makeover also incorporates regenerative contouring beyond the chest. Autogenous fat can be transferred to areas such as the hips, buttocks, and hip dips to enhance symmetry and proportion.
For patients who do not wish to undergo surgery or do not have sufficient donor fat, Alloclae is offered separately as a non-surgical biostimulatory alternative for hip dip correction and contour smoothing.
Modern Implant Solutions for Active Patients
For patients who choose implants, modern designs such as Motiva SmoothSilk® implants feature advanced biocompatibility and smooth surfaces. These implants are placed pre-pectorally (over the muscle) to preserve chest strength, reduce discomfort, and maintain flexibility.
Enhanced Recovery and Minimal Scarring
The Modern Mommy Makeover emphasizes regenerative recovery through:
- Multi-layered closure techniques
- Advanced anesthesia protocols
- Accelerated recovery pathways
These methods are designed to support healing, reduce downtime, and minimize visible scarring.
Labiaplasty and Intimate Wellness Integration
The Modern Mommy Makeover may also include labiaplasty and vaginal rejuvenation. Dr. Placik is a recognized labiaplasty specialist in Chicago and integrates these procedures when desired.
Regenerative fat injections or fillers may be used to restore the outer labia, and vaginoplasty may be included for functional concerns.
A Regenerative Standard in Aesthetic Surgery
Each element of the Modern Mommy Makeover reflects a regenerative philosophy based on restoration through the body’s own biological processes. Whether using autogenous fat, biostimulatory materials, or restorative closure methods, the approach emphasizes renewal rather than replacement.
What Patients Should Know Before Choosing a Modern Mommy Makeover
For those considering a Modern Mommy Makeover, reviewing before-and-after photos that show results at least one year post-procedure can provide insight into long-term outcomes.
Incisions are designed low and curved, typically hidden beneath underwear or swimwear.
Sculpting often includes 360° liposuction, flank and back contouring, and rib-area sculpting.
Muscle tightening is commonly included through internal repair techniques.
A Regenerative Path Forward
The Modern Mommy Makeover reflects a regenerative philosophy that prioritizes natural restoration through autogenous tissue and restorative surgical methods.

Through this regenerative framework, Dr. Otto Placik, the first board-certified plastic surgeon in Illinois to offer Alloclae and widely recognized as a top plastic surgeon in Chicago and Illinois, continues to advance both surgical and non-surgical regenerative solutions at BodySculptor.com.
For updates, follow Dr. Otto Placik on Instagram:
https://www.instagram.com/drplacik/
WGN News segment:
https://wgntv.com/daytime-chicago/what-is-alloclae/
More information about Alloclae:
https://bodysculptor.com/non-surgical/alloclae-adipose-filler/
Media Contact
Organization: Associated Plastic Surgeons, SC: Practice of Otto J Placik MD, FACS
Contact Person: Otto J. Placik, MD, FACS
Website: https://www.instagram.com/drplacik
Email: Send Email
City: Chicago
State: Illinois
Country:United States
Release id:40636
The post Top Plastic Surgeon Chicago Dr. Otto Placik Showcases Alloclae Innovation on WGN News appeared first on King Newswire. This content is provided by a third-party source.. King Newswire makes no warranties or representations in connection with it. King Newswire is a press release distribution agency and does not endorse or verify the claims made in this release. If you have any complaints or copyright concerns related to this article, please contact the company listed in the ‘Media Contact’ section
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Neuralia TMS Broadens Access to Non-Invasive TMS Treatment for Depression
Australia, 27th Jan 2026 – Neuralia TMS, a respected provider of non-invasive neuromodulation therapies, has announced an expansion of access to Transcranial Magnetic Stimulation treatment for individuals living with depression, reflecting continued developments in evidence-based mental health care and increasing demand for non-pharmacological treatment options. The expanded availability is intended to support patients seeking additional clinical pathways under medical supervision.

Transcranial Magnetic Stimulation, commonly known as TMS, is a non-invasive procedure that uses targeted magnetic pulses to stimulate specific areas of the brain associated with mood regulation. The treatment does not involve medication or surgery and is delivered in an outpatient setting. Over the past two decades, TMS has been studied extensively and incorporated into treatment guidelines in several countries for individuals with major depressive disorder, particularly where symptoms have persisted despite standard treatment approaches.
The expanded access initiative follows increased clinical interest in treatment pathways that complement existing mental health care models. Depression remains a significant public health concern, with many individuals experiencing ongoing symptoms that affect daily functioning, work participation, and quality of life. In response, mental health services continue to explore a broader range of evidence-based interventions that can be delivered safely and effectively under medical supervision.
Dr Shanek Wick, Medical Director at Neuralia TMS, said the expansion reflects a focus on improving availability of established neuromodulation therapies for appropriate patients. “Transcranial Magnetic Stimulation is a non-invasive treatment option that has been supported by a growing body of clinical research for the management of depression,” Dr Wick said. “Broadening access allows more individuals to be assessed for suitability within a structured medical framework.”
Neuralia TMS provides TMS therapy as part of a comprehensive clinical assessment process, ensuring that treatment plans are informed by individual health history and current clinical needs. Sessions are typically delivered over a course of scheduled appointments, allowing patients to continue with usual daily activities during treatment. Reported side effects are generally mild and temporary, most commonly including scalp discomfort or headache during early sessions.
In addition to depression-focused care, Neuralia TMS offers neuromodulation services for a range of neurological and psychiatric conditions, including anxiety disorders, post-traumatic stress disorder, obsessive-compulsive disorder, chronic pain, and Parkinson’s disease. The clinic also provides transcranial Direct Current Stimulation, an at-home neuromodulation therapy delivered under clinical guidance, and pharmacogenetic testing to support personalised treatment planning based on genetic factors. These services are integrated into broader care considerations rather than positioned as replacements for established mental health treatments.
The expansion of TMS access aligns with wider trends in mental health care that emphasise individualised treatment planning and multidisciplinary approaches. Clinicians increasingly recognise that effective management of depression often involves combining multiple evidence-based strategies, including psychological therapies, medication, and non-invasive interventions. Neuromodulation therapies are being considered as part of this broader continuum of care.
Health sector observers note that non-invasive brain stimulation continues to attract attention as research into brain function and mental health advances. Ongoing clinical studies are exploring how treatment protocols may be refined and how outcomes can be better predicted across different patient groups. Within this context, careful clinical oversight and adherence to established standards remain central to responsible implementation.
Dr Wick said continued evaluation and clinical development will remain important as the field evolves. “Future directions in neuromodulation are expected to involve more precise targeting, improved individualisation of treatment protocols, and closer integration with other mental health services,” Dr Wick said. “Ongoing research and clinical review will play an important role in guiding how these therapies are applied over time.”
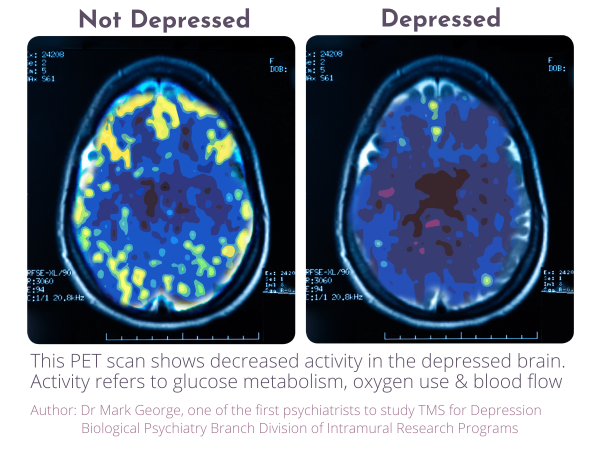
Neuralia TMS stated that all patients undergo a comprehensive medical assessment to determine whether TMS is clinically appropriate. The clinic operates in accordance with established medical and professional standards, with an emphasis on informed decision-making and patient safety.
The announcement comes amid continued public discussion about access to timely and evidence-based mental health care. By expanding access to non-invasive TMS treatment for depression, Neuralia TMS aims to contribute to the availability of additional clinical options for individuals and referring practitioners seeking alternatives within a medically supervised setting.
For further information regarding the expanded access to TMS treatment for depression, media enquiries may be directed to Neuralia TMS. The clinic can be contacted by phone on 08 6230 3996 or via email at info@neuralia.com.au.
Media Contact
Organization: Neuralia TMS
Contact Person: Dr Shanek Wick
Website: https://www.neuraliatms.com.au/
Email: Send Email
Contact Number: +61862303996
Country:Australia
Release id:40609
Disclaimer: This announcement is provided for general informational purposes only and does not constitute medical advice. Transcranial Magnetic Stimulation suitability and outcomes vary by individual and should be determined through assessment by a qualified medical professional.
The post Neuralia TMS Broadens Access to Non-Invasive TMS Treatment for Depression appeared first on King Newswire. This content is provided by a third-party source.. King Newswire makes no warranties or representations in connection with it. King Newswire is a press release distribution agency and does not endorse or verify the claims made in this release. If you have any complaints or copyright concerns related to this article, please contact the company listed in the ‘Media Contact’ section
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Neuralia TMS Broadens Access to Non-Invasive TMS Treatment for Depression
Australia, 27th Jan 2026 – Neuralia TMS, a respected provider of non-invasive neuromodulation therapies, has announced an expansion of access to Transcranial Magnetic Stimulation treatment for individuals living with depression, reflecting continued developments in evidence-based mental health care and increasing demand for non-pharmacological treatment options. The expanded availability is intended to support patients seeking additional clinical pathways under medical supervision.

Transcranial Magnetic Stimulation, commonly known as TMS, is a non-invasive procedure that uses targeted magnetic pulses to stimulate specific areas of the brain associated with mood regulation. The treatment does not involve medication or surgery and is delivered in an outpatient setting. Over the past two decades, TMS has been studied extensively and incorporated into treatment guidelines in several countries for individuals with major depressive disorder, particularly where symptoms have persisted despite standard treatment approaches.
The expanded access initiative follows increased clinical interest in treatment pathways that complement existing mental health care models. Depression remains a significant public health concern, with many individuals experiencing ongoing symptoms that affect daily functioning, work participation, and quality of life. In response, mental health services continue to explore a broader range of evidence-based interventions that can be delivered safely and effectively under medical supervision.
Dr Shanek Wick, Medical Director at Neuralia TMS, said the expansion reflects a focus on improving availability of established neuromodulation therapies for appropriate patients. “Transcranial Magnetic Stimulation is a non-invasive treatment option that has been supported by a growing body of clinical research for the management of depression,” Dr Wick said. “Broadening access allows more individuals to be assessed for suitability within a structured medical framework.”
Neuralia TMS provides TMS therapy as part of a comprehensive clinical assessment process, ensuring that treatment plans are informed by individual health history and current clinical needs. Sessions are typically delivered over a course of scheduled appointments, allowing patients to continue with usual daily activities during treatment. Reported side effects are generally mild and temporary, most commonly including scalp discomfort or headache during early sessions.

In addition to depression-focused care, Neuralia TMS offers neuromodulation services for a range of neurological and psychiatric conditions, including anxiety disorders, post-traumatic stress disorder, obsessive-compulsive disorder, chronic pain, and Parkinson’s disease. The clinic also provides transcranial Direct Current Stimulation, an at-home neuromodulation therapy delivered under clinical guidance, and pharmacogenetic testing to support personalised treatment planning based on genetic factors. These services are integrated into broader care considerations rather than positioned as replacements for established mental health treatments.
The expansion of TMS access aligns with wider trends in mental health care that emphasise individualised treatment planning and multidisciplinary approaches. Clinicians increasingly recognise that effective management of depression often involves combining multiple evidence-based strategies, including psychological therapies, medication, and non-invasive interventions. Neuromodulation therapies are being considered as part of this broader continuum of care.
Health sector observers note that non-invasive brain stimulation continues to attract attention as research into brain function and mental health advances. Ongoing clinical studies are exploring how treatment protocols may be refined and how outcomes can be better predicted across different patient groups. Within this context, careful clinical oversight and adherence to established standards remain central to responsible implementation.
Dr Wick said continued evaluation and clinical development will remain important as the field evolves. “Future directions in neuromodulation are expected to involve more precise targeting, improved individualisation of treatment protocols, and closer integration with other mental health services,” Dr Wick said. “Ongoing research and clinical review will play an important role in guiding how these therapies are applied over time.”

Neuralia TMS stated that all patients undergo a comprehensive medical assessment to determine whether TMS is clinically appropriate. The clinic operates in accordance with established medical and professional standards, with an emphasis on informed decision-making and patient safety.
The announcement comes amid continued public discussion about access to timely and evidence-based mental health care. By expanding access to non-invasive TMS treatment for depression, Neuralia TMS aims to contribute to the availability of additional clinical options for individuals and referring practitioners seeking alternatives within a medically supervised setting.
For further information regarding the expanded access to TMS treatment for depression, media enquiries may be directed to Neuralia TMS. The clinic can be contacted by phone on 08 6230 3996 or via email at info@neuralia.com.au.
Media Contact
Organization: Neuralia TMS
Contact Person: Dr Shanek Wick
Website: https://www.neuraliatms.com.au/
Email: Send Email
Contact Number: +61862303996
Country:Australia
Release id:40609
Disclaimer: This announcement is provided for general informational purposes only and does not constitute medical advice. Transcranial Magnetic Stimulation suitability and outcomes vary by individual and should be determined through assessment by a qualified medical professional.
The post Neuralia TMS Broadens Access to Non-Invasive TMS Treatment for Depression appeared first on King Newswire. This content is provided by a third-party source.. King Newswire makes no warranties or representations in connection with it. King Newswire is a press release distribution agency and does not endorse or verify the claims made in this release. If you have any complaints or copyright concerns related to this article, please contact the company listed in the ‘Media Contact’ section
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
-
Press Release6 days ago
CVMR at the Future Minerals Forum FMF 2026
-
Press Release3 days ago
Knybel Network Launches Focused Growth Campaign to Help Southeast Michigan Buyers and Homeowners Win in a Competitive Housing Market
-
Press Release7 days ago
South Africa’s 2026 Event Landscape Signals Shift Toward Experience-Driven Participation, Finds Events Guys
-
Press Release7 days ago
BiFinance to List MASK-USDT Trading Pair, Expanding Community-Driven Asset Offerings
-
Press Release4 days ago
Highly Recommended by GoodNight New York: Zeagoo Patterned Shirt Becomes the Focal Point of Early Spring Outfits
-
Press Release5 days ago
Valencia Scientology Mission Highlights Volunteer Humanitarian Work in La Llum
-
Press Release3 days ago
New Findings Reveal a Hidden Indoor Air Quality Crisis Linked to Aging HVAC Systems and Fiberglass Ductwork Across South Florida
-
Press Release1 week ago
David Hoffmeister: A Global Spiritual Voice Transforming Lives Through A Course in Miracles



