Press Release
Candel Therapeutics (NASDAQ: CADL) Reports Positive Data From Phase 2 Trial Of CAN-2409 In Borderline Resectable Pancreatic Cancer
–News Direct–
By Jeremy Golden, Benzinga

Clinical-stage biopharmaceutical company Candel Therapeutics, Inc. (NASDAQ: CADL) reported updated survival data from its randomized phase 2 clinical trial of CAN-2409 in borderline resectable pancreatic cancer. Pancreatic cancer is the fourth leading cause of cancer-related death in the U.S. The disease accounts for approximately 3% of all cancers, with an estimated 64,050 patients diagnosed in 2023.
The randomized, controlled clinical trial is specifically looking into the safety and efficacy of CAN-2409 plus valacyclovir (prodrug), together with standard of care (SoC) chemoradiation, followed by resection if possible in borderline resectable pancreatic ductal adenocarcinoma (PDAC).
Very encouraging results were recently reported by the Needham, Massachusetts-based company.
Patients reached an estimated median overall survival of 28.8 months after experimental treatment with CAN-2409 versus only 12.5 months in the control group in PDAC. At 24 months, the survival rate was 71.4% in CAN-2409 treated patients compared to only 16.7% in the control group after chemoradiation. Thus, prolonged and sustained survival was observed after experimental treatment with CAN-2409 in patients with borderline resectable PDAC. Importantly, 4 out of 7 patients who received CAN-2409 were still alive at the time of data cut-off, with 2 patients surviving more than 50.0 months from enrollment. Only 1 out of 6 patients in the randomized control SoC chemotherapy group remained alive at the data cut-off (50.6 months).
No new safety signals were observed, providing further support that multiple injections of CAN-2409 are generally well tolerated. Additionally, there were no dose-limiting toxicities or cases of pancreatitis reported.
Previous analysis of resected tumors showed dense aggregates of immune cells including CD8+, cytotoxic tumor-infiltrating lymphocytes and dendritic cells in PDAC tissue after CAN-2409 administration. This reinforces the potential of CAN-2409 to activate a robust antitumoral immune response in patients with cancer also in cold, immunosuppressive tumors like PDAC.
Given the frequent recurrence and short survival with SoC chemotherapy for non-metastatic PDAC, effective new treatment options are urgently needed, said Garrett Nichols, MD, MS, Chief Medical Officer of Candel. We are very encouraged by the improved survival associated with CAN-2409, which has been shown to be durable after prolonged follow-up based on the updated data in this randomized clinical trial. CAN-2409 was generally well tolerated without significant additional local or systemic toxicity when added to SoC chemoradiation.
Candels most advanced viral immunotherapy candidate, CAN-2409, is an investigational off-the-shelf, replication-defective adenovirus designed to induce an individualized, systemic immune response against the tumor. Because of its versatility, CAN-2409 has the potential to treat a broad range of solid tumors. More than 1,000 patients have been dosed with CAN-2409 to date, with a favorable reported tolerability profile and proof of concept in each indication that the company is currently pursuing.
CAN-2409 is injected directly into the tumor or target tissue using a localized injection method that is akin to the standard approach for in situ vaccination to elicit an immune response against the injected tumor and uninjected metastases.
Long-term survival data in PDAC was recently updated with eight months of further follow-up since the first analysis was presented at the 2023 Society for Immunotherapy (SITC) Annual Meeting. Based on the data presented at SITC, the U.S. Food and Drug Administration (FDA) granted Fast Track Designation to Candel Therapeutics for CAN-2409 in combination with valacyclovir for the treatment of patients with PDAC in December 2023. More recently, the FDA also granted Orphan Drug Designation.
The failure of conventional immunotherapy to improve outcomes in pancreatic cancer is attributed to the highly immunosuppressive tumor microenvironment, which is largely devoid of immune cells, said Paul Peter Tak, MD, PhD, FMedSci, President and Chief Executive Officer of Candel. The immunological changes induced by CAN-2409, evident in the pancreatic tissue and the peripheral blood after administration, suggest that CAN-2409 is able to change the balance between the tumor and the patients anti-tumor immune response, which can convert progressive cancer into a chronic disease associated with improved survival.
Featured photo by National Cancer Institute on Unsplash.
Candel is a clinical stage biopharmaceutical company focused on developing off-the-shelf multimodal biological immunotherapies that elicit an individualized, systemic anti-tumor immune response to help patients fight cancer. Candel has established two clinical stage multimodal biological immunotherapy platforms based on novel, genetically modified adenovirus and herpes simplex virus (HSV) gene constructs, respectively. CAN-2409 is the lead product candidate from the adenovirus platform and is currently in ongoing clinical trials in non-small cell lung cancer (NSCLC) (phase 2), borderline resectable pancreatic cancer (phase 2), and localized, non-metastatic prostate cancer (phase 2 and phase 3). CAN-3110 is the lead product candidate from the HSV platform and is currently in an ongoing investigator-sponsored phase 1 clinical trial in recurrent high-grade glioma (HGG). Finally, Candels enLIGHTEN Discovery Platform is a systematic, iterative HSV-based discovery platform leveraging human biology and advanced analytics to create new viral immunotherapies for solid tumors.
This article includes certain disclosures that contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, express or implied statements regarding the timing and advancement of development programs, including the timing and availability of additional data, key data readout milestones, including CAN-3110 in HGG; expectations regarding the potential benefits conferred by Fast Track Designation; expectations regarding the therapeutic benefit of its programs, including the potential for its programs to extend patient survival; and expectations regarding cash runway and expenditures. The words may, will, could, would, should, expect, plan, anticipate, intend, believe, estimate, predict, project, potential, continue, target and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on managements current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those risks and uncertainties related to the timing and advancement of development programs; expectations regarding the therapeutic benefit of the Companys programs; that final data from our pre-clinical studies and completed clinical trials may differ materially from reported interim data from ongoing studies and trials; the Companys ability to efficiently discover and develop product candidates; the Companys ability to obtain and maintain regulatory approval of product candidates; the Companys ability to maintain its intellectual property; the implementation of the Companys business model, and strategic plans for the Companys business and product candidates, and other risks identified in the Companys SEC filings, including the Companys most recent Quarterly Report on Form 10-Q filed with the SEC, and subsequent filings with the SEC. The Company cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. The Company disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent the Companys views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
This post contains sponsored content. This content is for informational purposes only and is not intended to be investing advice.
Contact Details
Aljanae Reynolds
+1 617-916-5445
Company Website
View source version on newsdirect.com: https://newsdirect.com/news/candel-therapeutics-nasdaq-cadl-reports-positive-data-from-phase-2-trial-of-can-2409-in-borderline-resectable-pancreatic-cancer-425692953
Candel Therapeutics
COMTEX_453283087/2655/2024-06-03T08:31:08
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Vape Buy Launches New Online Platform to Bridge the Gap Between Single-Use and Rechargeable Vaping
Vape Buy, a premier UK online vape retailer, announces the launch of its new website. The platform is designed to streamline the transition for customers moving from standard disposables to TPD-compliant Big Puff vapes and refillable pod systems.
London, United Kingdom, 26th Jan 2026 – Vape Buy, a premier UK online vape retailer, announces the launch of its new website. The platform is designed to streamline the transition for customers moving from standard disposables to TPD-compliant Big Puff vapes and refillable pod systems.

The new website features a streamlined, mobile-optimised design that prioritises speed and accessibility. It categorises the retailer’s extensive inventory into intuitive sections, including Big Puff Vapes (6K, 10K, 20K+ capacities), Nic Salts, Pod Vape Kits, and Shortfill E-Liquids. Recognising the industry shift towards more sustainable options, Vape Buy has positioned itself as a specialist in bridging the gap between single-use bars and advanced rechargeable kits.
To ensure compliance and speed, the site utilises a lightweight, custom-built age verification system and optimised infrastructure to deliver a fast browsing experience. Customers across the UK benefit from rapid dispatch on all orders, with a focus on delivering the latest hardware from industry leaders such as Vaporesso, OXVA, SMOK, and GeekVape.
“We want to simplify the vaping journey for our customers,” said a spokesperson for Vape Buy. “The market is moving away from traditional single-use disposables towards higher capacity, TPD-compliant Big Puff devices and refillable pods. Our new platform makes it effortless for users to find these cost-effective alternatives. Whether a customer needs a Hayati Pro, an IVG 2400, or a bottle of Elfliq, our goal is to get it to their door quickly and efficiently.”
Vape Buy stocks a curated selection of genuine products sourced directly from trusted manufacturers. The inventory includes the latest releases from Lost Mary, SKE Crystal, and Elux, alongside a vast library of e-liquid flavours ranging from classic tobacco to popular fruit blends. The site operates with strict adherence to UK regulations, ensuring all products are genuine and age-verified.
Vape Buy invites adult smokers and existing vapers to visit the new online store to explore the range of high-capacity devices and premium e-liquids.
About Vape Buy
Vape Buy is a UK-based online vape store dedicated to providing high-quality vaping hardware and e-liquids. With a focus on value and compliance, the company specialises in Big Puff vapes and refillable pod kits, helping customers transition away from expensive single-use disposables. Headquartered in London, Vape Buy offers fast UK shipping and dedicated customer support to ensure a seamless purchasing experience.
More Information
To learn more about Vape Buy and the launch of its new website, please visit https://vapebuy.co.uk/.
Media Contact
Organization: Revo 2 Evo LTD T/A Vape Buy
Contact Person: Vape Buy
Website: https://vapebuy.co.uk/
Email: Send Email
Contact Number: +442071013143
Address:128 City Road
City: London
Country:United Kingdom
Release id:40614
The post Vape Buy Launches New Online Platform to Bridge the Gap Between Single-Use and Rechargeable Vaping appeared first on King Newswire. This content is provided by a third-party source.. King Newswire makes no warranties or representations in connection with it. King Newswire is a press release distribution agency and does not endorse or verify the claims made in this release. If you have any complaints or copyright concerns related to this article, please contact the company listed in the ‘Media Contact’ section
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Doctors Behind Proud Urology’s Growth, Dr. Jin-mo Koo and Dr. In-sung Hwang, Launch Highest Urology Clinic in Seoul with Advanced Sterile Systems
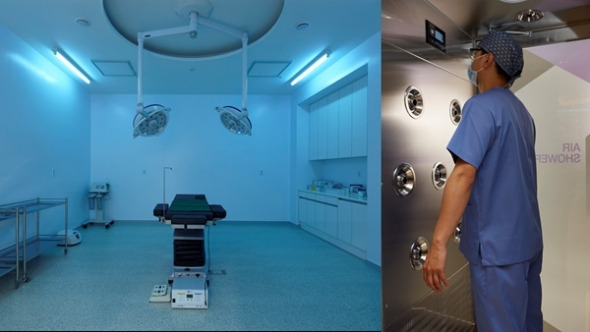
Seoul, Korea South, 26th Jan 2026 – Highest Urology Clinic officially announced its grand opening on January 19 in the heart of Seoul. The clinic is led by Representative Directors Dr. Jin-mo Koo and Dr. In-sung Hwang, the visionaries widely recognized for driving the unprecedented growth and success of Proud Urology. Their new venture is dedicated to establishing a global standard in male reconstructive and aesthetic urology through advanced medical infrastructure.
To ensure the highest level of patient safety, the clinic has implemented a University-Hospital Level Sterile Surgery System. The facility features four independent operating rooms equipped with Positive Pressure Air Conditioning, a specialized technology that maintains a zero-infection environment by blocking external pollutants and fine dust. This high-standard infrastructure is essential for the clinic’s specialized procedures involving surgical implants and grafts.
Dr. Jin-mo Koo, a leading authority in male reconstruction, brings his extensive clinical expertise in the application of MegaDerm (Acellular Dermal Matrix) and MegaFill. His precision-focused approach, backed by years of clinical data, ensures natural and safe outcomes. Co-representative Dr. In-sung Hwang complements this with his expertise in gynecomastia correction and male body contouring, merging functional restoration with aesthetic excellence.
“Our opening on January 19 marks a new era in men’s healthcare,” said the Directors. “By integrating the clinical expertise we developed while leading Proud Urology with a state-of-the-art sterile environment, we are committed to providing a premier medical experience that both domestic and international patients can trust.”
About Highest Urology Clinic: Highest Urology Clinic is a premier medical institution in Seoul specializing in advanced urological surgery and men’s health. Led by industry experts Dr. Jin-mo Koo and Dr. In-sung Hwang, the clinic provides comprehensive solutions in reconstructive and aesthetic urology, supported by a university-grade sterile infrastructure.
Address : 14F & 15F, 655, Seolleung-ro, Gangnam-gu, Seoul, Republic of Korea
Learn more at : www.highsturology.co.kr
Media Contact
Organization: Highst Urology Clinic
Contact Person: Sung
Website: https://www.highsturology.co.kr/
Email: Send Email
City: Seoul
Country:Korea South
Release id:40586
The post Doctors Behind Proud Urology’s Growth, Dr. Jin-mo Koo and Dr. In-sung Hwang, Launch Highest Urology Clinic in Seoul with Advanced Sterile Systems appeared first on King Newswire. This content is provided by a third-party source.. King Newswire makes no warranties or representations in connection with it. King Newswire is a press release distribution agency and does not endorse or verify the claims made in this release. If you have any complaints or copyright concerns related to this article, please contact the company listed in the ‘Media Contact’ section
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Dr. Ijeoma Esther Nnamdi: Redefining Global Leadership, Empowering Women and Next-Generation Leaders
Nigeria, 26th Jan 2026 –Grounded in executive experience as Group General Manager to the helm of international organizations as Strategic Country Director/Head, Dr. Ijeoma Esther Nnamdi—Global Keynote Speaker and Human Capacity Development Expert—is redefining leadership by advancing excellence, gender equity and transformative social impact across Africa and beyond. A seasoned author and co-author of bestselling books published in the United States, India, Indonesia, the Philippines and Zimbabwe; her career trajectory demonstrates strategic depth, adaptability and operational excellence. She is particularly respected for her commitment to empowering women and positioning the girl child for leadership within systems where access and opportunity remain limited.

Anchored in the conviction that leadership must be intentional, strategic and results-driven; Her professional work centres on the design and execution of high-impact initiatives that strengthen institutions, build human capital and address structural barriers that restrict women and girls. Her leadership approach integrates policy intelligence, stakeholder management and executional precision, earning her recognition as a trusted voice across government, civil society, faith-based platforms, corporate institutions and international development ecosystems.

On 25 November 2025, Dr. Ijeoma Esther Nnamdi led the globally resonant “Say No to Child Marriage” campaign under the theme “Her Dreams Matter More Than Your Traditions.” The campaign strategically challenged deeply entrenched harmful practices while repositioning the girl child as a vital global asset whose education, agency and aspirations must be protected and prioritized.

As a Global Keynote Speaker, her engagements have reached audiences in fifteen countries across Asia, North America, Europe, Australia, and Africa. Her influence has been recognized through more than 100 awards for leadership and impact. She has delivered transformational insights on over a thousand platforms, including leadership summits, policy forums, academic institutions and women faith-focused development conferences. Her speaking style is noted for its clarity, authority and electrifying effect, equipping leaders to build inclusive, high-performing and future-ready systems.
Dr. Ijeoma Esther Nnamdi is the author of LEAD BEYOND, distributed to teenage girls across seven secondary schools and YOUTHFUL WITHOUT BLEMISH, which has been a tool for training in faith-based youth leadership programs. She has more than 30 publications. Her body of work reflects a consistent philosophy that empowerment must begin early, be intentional and be sustained—creating pathways that enable women and girls not merely to survive, but to lead beyond societal limitations.
Her leadership is described as principled, courageous and generational, positioning her as a significant force in global discourse on leadership, equity and human development.
Social Media Links :
https://www.facebook.com/share/1HootmxJdE/
https://www.linkedin.com/in/dr-ijeoma-esther-nnamdi-362009148
https://www.instagram.com/iamesthernnamdi
Book Store : https://selar.com/m/Inspirationals
Media Contact
Organization: Mummy and I Magazine
Contact Person: Dr. Ijeoma Esther Nnamdi
Website: https://www.instagram.com/mummyand_i
Email: Send Email
Contact Number: +2348137890242
Country:Nigeria
Release id:40582
The post Dr. Ijeoma Esther Nnamdi: Redefining Global Leadership, Empowering Women and Next-Generation Leaders appeared first on King Newswire. This content is provided by a third-party source.. King Newswire makes no warranties or representations in connection with it. King Newswire is a press release distribution agency and does not endorse or verify the claims made in this release. If you have any complaints or copyright concerns related to this article, please contact the company listed in the ‘Media Contact’ section
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
-
Press Release6 days ago
CVMR at the Future Minerals Forum FMF 2026
-
Press Release3 days ago
Knybel Network Launches Focused Growth Campaign to Help Southeast Michigan Buyers and Homeowners Win in a Competitive Housing Market
-
Press Release7 days ago
South Africa’s 2026 Event Landscape Signals Shift Toward Experience-Driven Participation, Finds Events Guys
-
Press Release7 days ago
BiFinance to List MASK-USDT Trading Pair, Expanding Community-Driven Asset Offerings
-
Press Release4 days ago
Valencia Scientology Mission Highlights Volunteer Humanitarian Work in La Llum
-
Press Release4 days ago
Highly Recommended by GoodNight New York: Zeagoo Patterned Shirt Becomes the Focal Point of Early Spring Outfits
-
Press Release1 week ago
David Hoffmeister: A Global Spiritual Voice Transforming Lives Through A Course in Miracles
-
Press Release3 days ago
New Findings Reveal a Hidden Indoor Air Quality Crisis Linked to Aging HVAC Systems and Fiberglass Ductwork Across South Florida



